


 微信在线咨询
微信在线咨询

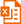
| 产品编号 | 产品名称 | 产品包装 | 产品价格 |
| C4102-100μl | Lenti-EF1α-Cre-P2A-EGFP-Puro (10^8TU/ml) | 100μl | 621.00元 |
| C4102-1ml | Lenti-EF1α-Cre-P2A-EGFP-Puro (10^8TU/ml) | 10×100μl | 4142.00元 |
Lenti-EF1α-Cre-P2A-EGFP-Puro,即Lentivirus expressing Cre recombinase, EGFP and puromycin,是碧云天研发的、可以在大多数哺乳动物细胞(包括原代细胞和干细胞)中通过EF1α启动子启动表达Cre recombinase (Cre重组酶)和EGFP的重组慢病毒。含有预先插入loxP位点的条件性敲除(Conditional knockout)细胞或组织在被Lenti-EF1α-Cre-P2A-EGFP-Puro病毒感染后,其表达的Cre Recombinase可以导致两个loxP位点间的基因重组,从而实现目的基因的敲除。
EF1α启动子是一种来源于延伸因子1α (elongation factor 1 alpha, EF1A)基因的强哺乳动物表达启动子,可在多种细胞中稳定驱动其下游基因的组成型表达,也可以用于干细胞、原代细胞、造血细胞等。
Cre重组酶是来源于大肠杆菌噬菌体P1的一种I型拓朴异构酶(Type I topoisomerase),也是一种酪氨酸重组酶(tyrosine recombinase),能识别34bp的loxP位点(两端为两个13bp的反向重复序列(inverted repeats),中间是8bp的间隔区(图1),并能催化loxP位点之间的DNA发生重组;重组产物根据loxP位点的位置和相对方向的不同而不同,两个含单loxP位点的DNA将发生融合:两个同方向的loxP位点间的DNA将以环状形式被切割,而两个反向loxP位点间的DNA序列将被翻转(图2) [1, 2]。
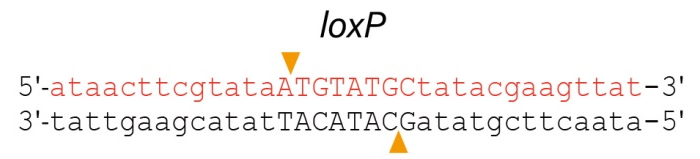
图1. loxP位点序列图。Cre重组酶与两端13bp反向重复序列(小写字母)结合,中间是8bp不对称中心间隔区(大写字母),箭头所示为Cre重组酶的酶切位点。
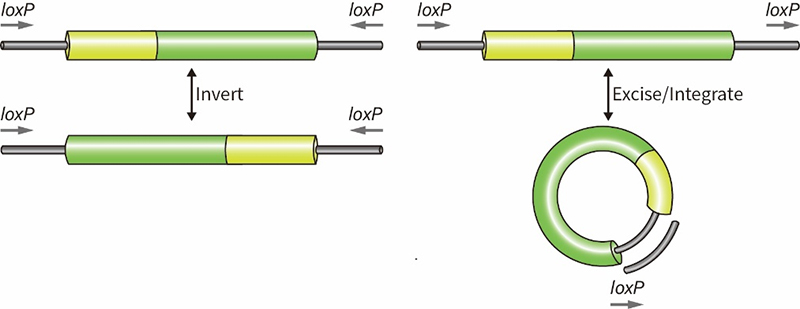
图2. Cre-loxP位点特异性重组示意图。
本慢病毒中的Cre重组酶编码序列和EGFP的编码序列之间含有P2A肽序列。P2A是一个可以被理解为含有19个氨基酸残基(ATNFSLLKQAGDVEENPGP)的“自剪切”小肽。但实际的过程并不是发生自剪切,而是使核糖体跳过P2A等2A元件C端的甘氨酸和脯氨酸肽键的合成而发挥作用,最终导致2A序列末端和下游产物分离。上游目的基因表达蛋白的C端将会添加一些额外的P2A残基(GSGATNFSLLKQAGDVEENPG),而下游蛋白的N端将会有额外的脯氨酸。在P2A肽的N端加入GSG序列,可提高剪切效率[3, 4]。通过显微镜下观察EGFP蛋白的表达,可确定Cre重组酶的表达。本产品表达的Cre重组酶和嘌呤霉素抗性基因的正常功能均不受额外的氨基酸影响。
本慢病毒含有EGFP和嘌呤霉素(puromycin)抗性基因,因此病毒感染细胞后可以使用流式细胞仪进行分选或使用嘌呤霉素筛选稳定细胞株。
碧云天Lenti-EF1α-Cre-P2A-EGFP-Puro感染HEK293T细胞的效果如图3所示。
图3. 碧云天Lenti-EF1α-Cre-P2A-EGFP-Puro感染HEK293T细胞的效果图。96孔板每孔10,000个细胞,培养过夜后用相应的剂量病毒进行感染,病毒感染72小时后荧光显微镜实拍相同大小视野效果图。实际检测效果会因检测仪器、实验条件的不同而存在差异,本图仅供参考。
Lenti-EF1α-Cre-P2A-EGFP-Puro感染Loxp-STOP-loxP-mCherry HeLa细胞的效果如图4所示。
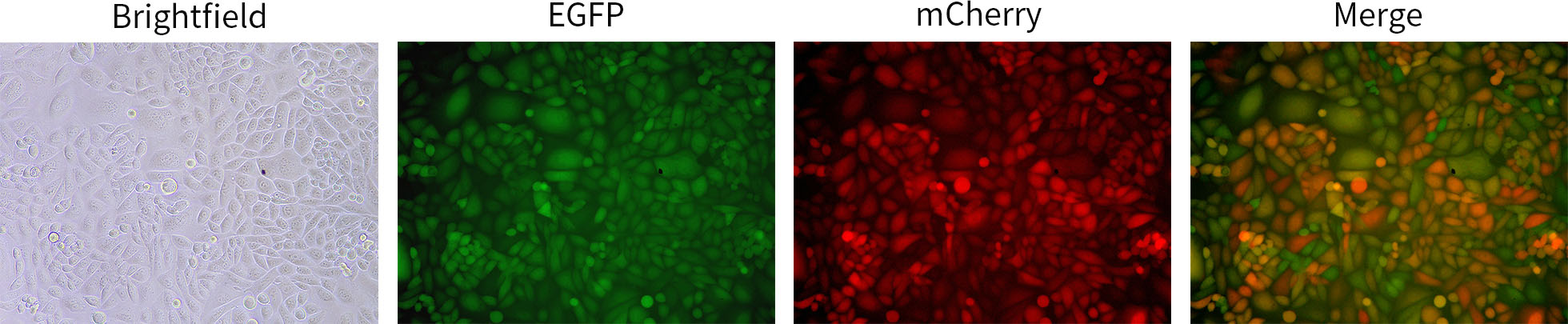
图4. 碧云天Lenti-EF1α-Cre-P2A-EGFP-Puro感染Loxp-STOP-loxP-mCherry HeLa细胞(C8012)的效果图。96孔板每孔接种10,000个Loxp-STOP-loxP-mCherry HeLa细胞,培养过夜后按照MOI=5进行感染,病毒感染72小时后荧光显微镜实拍相同位置视野效果图。Lenti-EF1α-Cre-P2A-EGFP-Puro感染细胞后因表达EGFP而呈现绿色荧光;Cre重组酶的表达导致Loxp-STOP-loxP-mCherry HeLa细胞中原本被阻断的mCherry被激活表达,从而使细胞呈现红色荧光。实际检测效果会因检测仪器、实验条件的不同而存在差异,本图仅供参考。
慢病毒感染细胞后会将目的基因随机整合到基因组DNA上,因而可以长期稳定地表达目的蛋白,并且几乎可以感染所有哺乳动物细胞,在很多不分裂的细胞中也可以长期稳定表达,广泛应用于细胞和动物实验。
碧云天的Lenti-EF1α-Cre-P2A-EGFP-Puro是复制缺陷型慢病毒。其3' LTR的增强子功能发生缺失,形成了自失活(self-inactivating) 3' LTR,在感染普通的细胞后不能进行复制和扩增,从而有效降低了本产品在活体生物中的风险。
碧云天推荐的慢病毒感染不同种类体外培养细胞的MOI值参见下表。
| Cell line | Tissue | Cancer/cell type | Species | MOI |
| A431 | Epithelial | Carcinoma | Human | 5 |
| A549 | Lung | Carcinoma | Human | 5 |
| Astrocytes | Nervous system | Primary | Human | 1 |
| B16-F10 | Epithelial | Melanoma, metastatic | Mouse | 5 |
| BMM | Bone Marrow | Primary | Human | 8 |
| BxPC-3 | Pancreas, epithelial | Adenocarcinoma | Human | 10 |
| H3255 | Lung | Carcinoma, NSCLC | Human | 10 |
| HCT116 | Colon | Carcinoma | Human | 5 |
| HeLa | Cervix | Carcinoma, epithelioid | Human | 3 |
| HEK293T | Kidney | Tumor | Human | 5 |
| Hepa1-6 | Liver | Carcinoma | Mouse | 3 |
| HMVEC | Endothelial | Endothelial, microvascular | Human | 100 |
| HT-29 | Colon | Adenocarcinoma | Human | 3 |
| HUVEC | Umbilicus | Endothelial cells | Human | 100 |
| Jurkat | Blood | Leukemia, Acute T cell | Human | 10 |
| LLC-1 | Lung | Carcinoma | Mouse | 6 |
| LNCaP | Prostate | Carcinoma | Human | 5 |
| MM200 | Skin | Melanoma | Human | 5 |
| MCF-7 | Breast | Adenocarcinoma | Human | 2 |
| MDA-MB-231 | Breast | Adenocarcinoma | Human | 1 |
| MM-AN | Skin | Melanoma, metastatic | Human | 16 |
| MMC | Breast | Carcinoma | Mouse | 4 |
| MRC-5 | Lung, embryonic | Fibroblasts | Human | 1 |
| NB4 | Blood | Leukemia, acute promyelocytic | Human | 10 |
| PC12 | Adrenal gland | Pheochromocytoma | Rat | 20 |
| SKOV-3 | Ovary | Adenocarcinoma | Human | 15 |
| U-2 OS | Bone | Osteosarcoma | Human | 5 |
实验室常用的逆转录病毒(Retrovirus)、慢病毒(Lentivirus)、腺相关病毒(AAV)和腺病毒(Adenovirus)的主要特征之间的比较和差别参见下表。具体的特定病毒的一些特征和下表相比可能会有一定差异。
| Retrovirus | Lentivirus | AAV | Adenovirus | |
| Genome | ssRNA(+) | ssRNA(+) | ssDNA | dsDNA |
| Coat | Enveloped | Enveloped | Naked | Naked |
| Particle size | 90-100nm | 90-100nm | 20-30nm | 60-90nm |
| Genome size | 7-10kb | 9kb | 5kb | 38-39kb |
| Genome integration | Yes | Yes | No | No |
| Packaging capacity | 2.5-5kb | 2.5-6kb | 2.5-4.5kb | 3-8kb |
| Infection tropism | Dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells |
| Relative Transduction Efficiency | ND | 70% | 70% | 100% |
| Expression started | 48-72h | 48-72h | 72-96h | 24-48h |
| Expression duration | > 2 months | > 2 months | > 6 months | 3-4 weeks |
| Expression level | Medium | Medium | Medium | High |
| Immune response | Low | Low | Very low | High |
| In vivo safety | Medium | Medium | High | Low |
| Titer before concentration (IFU/ml) | 106 | 107 | 1011 | 107 |
| Titer after concentration (IFU/ml) | ND | 108 | 0.5-1×1013 | 1010 |
| Able to obtain high MOI | No (≤ 10 copies integrated) | No (≤ 10 copies integrated) | Yes | Yes |
| Biosafety level | BSL-2 | BSL-2 | BSL-1 | BSL-2 |
本产品的滴度不低于10^8TU/ml,适合细胞实验或活体动物实验。TU, transduction unit,即转导单位。TU通过本产品感染HEK293T细胞72小时后,抽提细胞基因组DNA进行qPCR测定。MOI (Multiplicity of Infection)是病毒感染细胞时,病毒数量与细胞数量的比值。使用10^8TU/ml的本产品,如果按照5 MOI感染6孔板的细胞,每孔50万细胞计算,1ml共可以感染40个孔;如果按照5 MOI感染24孔板的细胞,每孔10万细胞计算,1ml共可以感染200个孔。如果MOI值提高,那么相应可以感染的孔数会减少;如果MOI值下调,那么相应可以感染的孔数会增加。
包装清单:| 产品编号 | 产品名称 | 包装 |
| C4102-100μl | Lenti-EF1α-Cre-P2A-EGFP-Puro (10^8TU/ml) | 100μl |
| C4102-1ml | Lenti-EF1α-Cre-P2A-EGFP-Puro (10^8TU/ml) | 10×100μl |
| - | 说明书 | 1份 |
-80℃保存,一年有效。-20℃保存,1-2个月内有效。4℃保存,一周内有效。
注意事项:反复冻融会降低病毒滴度,如有必要请在收到本产品后分装保存。分装时必须在冰浴上进行。病毒融解后,如果在一周内使用,可以放于4℃,但须注意4℃存放时间越长,滴度下降越明显。如果-80℃保存时间超过一年,可能会导致滴度下降,此时建议重新测定病毒滴度。
本产品使用前请仔细阅读附录1《慢病毒使用安全规范》。本产品生物安全等级为Biosafety Level 2 (BSL-2),在按照常规的微生物实验操作要求进行操作(Standard microbiological practices)的基础上,还需要注意限制接触、生物危害提示、显著的警示标识、并制定相应的安全规范。
病毒操作中应注意有效防护,绝对禁止在生物安全柜内有任何皮肤直接暴露的情况。实验完成后,请及时清洗双手。严禁直接接触病毒,如意外接触,请及时用清水冲洗,并适当用70%乙醇对皮肤进行消毒。
任何接触过病毒的材料、试剂、样品,应经消毒处理,可以采用1%的SDS溶液、或84消毒液(1:20)浸泡30分钟以上,或121℃高压灭菌30分钟。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
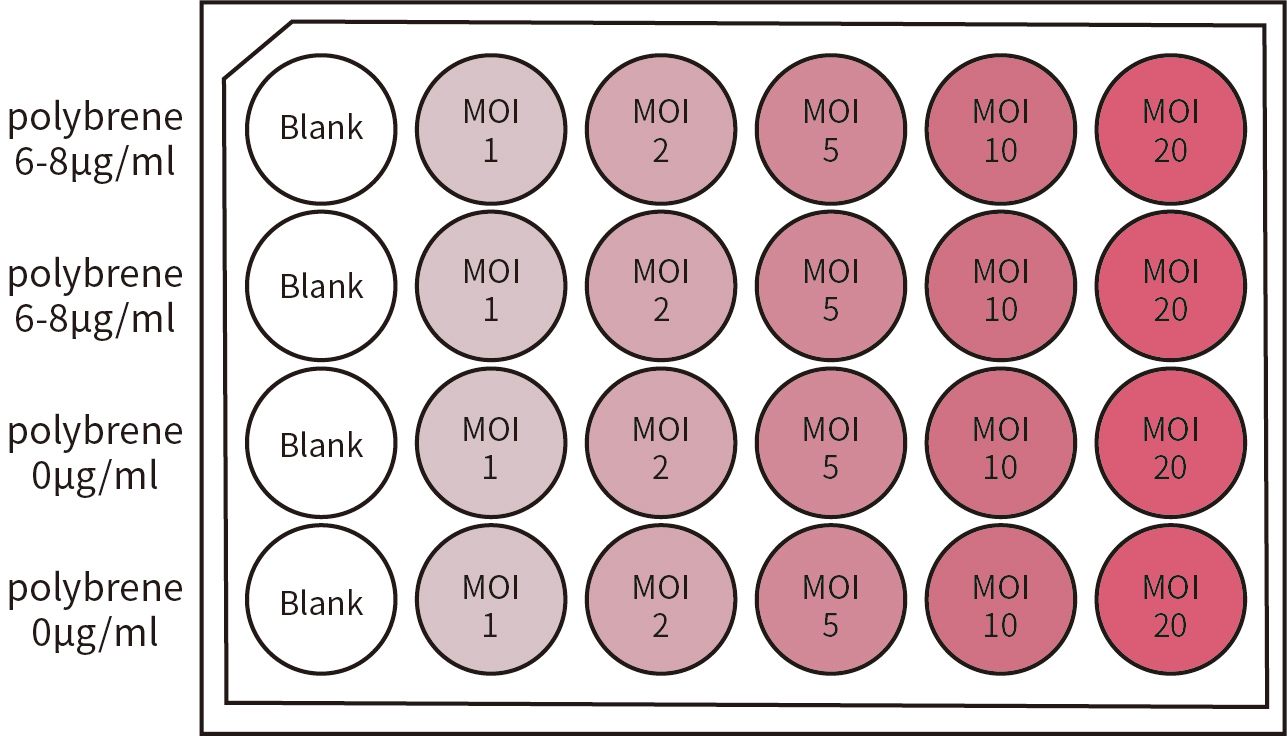 图5.细胞感染MOI值预实验设计分组。MOI依次设置为:1、2、5、10、20。并同时设置加含6-8μg/ml polybrene的培养液实验组,不含polybrene的培养液实验组及Blank组(空白对照组)。Blank组可作为参照,以检验细胞生长状态。本图中实验设置仅供参考,用户应根据实际情况自行适当调整。
图5.细胞感染MOI值预实验设计分组。MOI依次设置为:1、2、5、10、20。并同时设置加含6-8μg/ml polybrene的培养液实验组,不含polybrene的培养液实验组及Blank组(空白对照组)。Blank组可作为参照,以检验细胞生长状态。本图中实验设置仅供参考,用户应根据实际情况自行适当调整。| BSL | Agents | Practices | Primary Barriers andSafety Equipment | Facilities(Secondary Barriers) |
| 1 | Not known to consistently cause diseases in healthy adults | Standard microbiological practices | ■ No primary barriers required. ■ PPE: laboratory coats and gloves; eye, face protection, as needed |
Laboratory bench and sink required |
| 2 | ■ Agents associated with human disease ■ Routes of transmission include percutaneous injury, ingestion, mucous membrane exposure |
BSL-1 practice plus: ■ Limited access ■ Biohazard warning signs ■ “Sharps” precautions ■ Biosafety manual defining any needed waste decontamination or medical surveillance policies |
Primary barriers: ■ BSCs or other physical containment devices used for all manipulations of agents that cause splashes or aerosols of infectious materials ■ PPE: Laboratory coats, gloves, face and eye protection, as needed |
BSL-1 plus: ■ Autoclave available |
| 3 | Indigenous or exotic agents that may cause serious or potentially lethal disease through the inhalation route of exposure | BSL-2 practice plus: ■ Controlled access ■ Decontamination of all waste ■ Decontamination of laboratory clothing before laundering |
Primary barriers: ■ BSCs or other physical containment devices used for all open manipulations of agents ■ PPE: Protective laboratory clothing, gloves, face, eye and respiratory protection, as needed |
BSL-2 plus: ■ Physical separation from access corridors ■ Self-closing, double-door access ■ Exhausted air not recirculated ■ Negative airflow into laboratory ■ Entry through airlock or anteroom ■ Hand washing sink near laboratory exit |
| 4 | ■ Dangerous/exotic agents which post high individual risk of aerosol-transmitted laboratory infections that are frequently fatal, for which there are no vaccines or treatments ■ Agents with a close or identical antigenic relationship to an agent requiring BSL-4 until data are available to redesignate the level ■ Related agents with unknown risk of transmission |
BSL-3 practices plus: ■ Clothing change before entering ■ Shower on exit ■ All material decontaminated on exit from facility |
Primary barriers: ■ All procedures conducted in Class III BSCs or Class I or II BSCs in combination with full-body, air-supplied, positive pressure suit |
BSL-3 plus: ■ Separate building or isolated zone ■ Dedicated supply and exhaust, vacuum, and decontamination systems ■ Other requirements outlined in the text |
| 产品编号 | 产品名称 | 包装 |
| C4105-100μl | Lenti-EF1α-Cre-P2A-mCherry-Puro (10^8TU/ml) | 100μl |
| C4105-1ml | Lenti-EF1α-Cre-P2A- mCherry-Puro (10^8TU/ml) | 1ml |
| C8011 | LoxP-STOP-loxP-EGFP HeLa Cells | 1支/瓶 |
| C8012 | LoxP-STOP-loxP-mCherry HeLa Cells | 1支/瓶 |
| D0509S | Cre Recombinase | 50U |
| D0509M | Cre Recombinase | 250U |
| D0509L | Cre Recombinase | 1000U |
| D2607-1μg | pCMV-Cre-mCherry | 1μg |
| D2607-100μg | pCMV-Cre-mCherry | 100μg |
| D2608-1μg | pCMV-Cre-EGFP | 1μg |
| D2608-100μg | pCMV-Cre-EGFP | 100μg |
| D0508S | 基因组编辑突变检测试剂盒 | 25次 |
| D0508M | 基因组编辑突变检测试剂盒 | 100次 |
| D7080S | T7 Endonuclease I (CRISPR等基因突变鉴定用) | 250U |
| D7080M | T7 Endonuclease I (CRISPR等基因突变鉴定用) | 1250U |
| D7080L | T7 Endonuclease I (CRISPR等基因突变鉴定用) | 5000U |









 微信在线咨询
微信在线咨询












