


 微信在线咨询
微信在线咨询

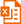
| 产品编号 | 产品名称 | 产品包装 | 产品价格 |
| C4007-100μl | Lenti-CMV-CD9-EGFP (10^9TU/ml,外泌体示踪用) | 100μl | 2888.00元 |
| C4007-500μl | Lenti-CMV-CD9-EGFP (10^9TU/ml,外泌体示踪用) | 100μl×5 | 11588.00元 |
Lenti-CMV-CD9-EGFP,即Lentivirus expressing CD9-EGFP fusion protein,是碧云天自行研发的一种可以在大多数哺乳动物细胞(包括原代细胞和干细胞)中通过CMV启动子表达人源CD9-EGFP融合蛋白的重组慢病毒。本产品可用于外泌体的示踪研究,用于研究外泌体的形成、分泌、靶向和运输机制。本产品带有嘌呤霉素(Puromycin)抗性基因,感染细胞后可以使用嘌呤霉素筛选稳定株。
外泌体(Exosome)是膜包裹的细胞外囊泡(Extracellular vesicles, EVs),直径约为40-160nm,具有脂质双分子层结构,天然存在于血液、尿液、脑脊液,以及体外培养细胞的上清液中[1],几乎所有类型的细胞都可以产生并释放外泌体[2]。如图1所示,细胞膜内吞(Endocytosis)依次形成初级内体(Early sorting endosome, ESE)、次级内体(Late sorting endosome, LSE)和多囊泡体(Multivesicular body, MVB),其中多囊泡体包含腔内囊泡(Intraluminal vesicles, ILVs)。多囊泡体与细胞膜融合形成外泌体,外泌体携带多种来自其母体细胞的成分(包括核酸、蛋白质、脂类和代谢物等)释放到胞外基质中[3]。外泌体可以被附近或远距离的细胞识别和融合,是细胞间进行相互调控的重要媒介,参与了癌症、神经退行性病变和炎症性疾病等多种疾病的发病过程,影响细胞多方面的功能[4-5]。

图1. 外泌体的形成原理及其携带的母体细胞成分示意图。
外泌体标志蛋白(Protein biomarkers for exosome),也称外泌体蛋白标志物(Protein markers for exosome),可以用于鉴定、观察、标记和纯化外泌体。据外泌体据库(ExoCarta, http://www.exocarta.org)统计来自不同生物、细胞类型和体液的外泌体中已发现9769种蛋白质,ExoCarta列出了100种常见外泌体蛋白标志物(http://exocarta.org/exosome_markers_new),其中CD9、CD63、CD81、Alix、Flotillin、Ceramide和Tumor susceptibility gene 101 (TSG101)是最常用的外泌体蛋白标志物(如图2所示[3])。CD9在人体中由CD9基因编码的蛋白质抗原,是跨膜四超家族(Transmembrane 4 superfamily)成员之一,为4次跨膜蛋白,大量存在于细胞外囊泡的表面,也会出现在普通的细胞膜表面。CD9是最常用的外泌体蛋白标志物之一,可以调节细胞黏附和迁移,同时还可以触发血小板活化和聚集,被认为是血小板活化标志物[6]。

图2. 常见的外泌体蛋白标志物示意图。
Lenti-CMV-CD9-EGFP感染HEK293T细胞后的表达效果如图3所示。
图3. 碧云天生产的Lenti-CMV-CD9-EGFP (C4007)感染HEK293T细胞的效果图。96孔板每孔10,000个细胞,培养过夜后用相应的病毒TU数进行感染,病毒感染72小时后荧光显微镜实拍相同大小视野效果图。实际检测效果会因检测仪器、实验条件的不同而存在差异,本图仅供参考。
慢病毒感染细胞后会将目的基因随机整合到基因组DNA上,因而可以长期稳定地表达目的蛋白,并且几乎可以感染所有哺乳动物细胞,在很多不分裂的细胞中也可以长期稳定表达,广泛应用于细胞和动物实验。
本产品表达嘌呤霉素抗性基因,因此本产品感染培养的细胞后可以使用嘌呤霉素筛选稳定表达的细胞株以用于后续研究。
碧云天的Lenti-CMV-CD9-EGFP是复制缺陷型慢病毒。其3' LTR的增强子功能发生缺失,形成了自失活(Self-inactivating) 3' LTR,并且5' LRT中的U3区域替换成CMV启动子,在感染普通的细胞后不能进行复制和扩增,从而有效降低了本产品在活体生物中的风险。
碧云天推荐的慢病毒感染不同种类体外培养细胞的MOI值参见下表。
| Cell line | Tissue | Cancer/cell type | Species | MOI |
| A431 | Epithelial | Carcinoma | Human | 5 |
| A549 | Lung | Carcinoma | Human | 5 |
| Astrocytes | Nervous system | Primary | Human | 1 |
| B16-F10 | Epithelial | Melanoma, metastatic | Mouse | 5 |
| BMM | Bone Marrow | Primary | Human | 8 |
| BxPC-3 | Pancreas, epithelial | Adenocarcinoma | Human | 10 |
| H3255 | Lung | Carcinoma, NSCLC | Human | 10 |
| HCT116 | Colon | Carcinoma | Human | 5 |
| HeLa | Cervix | Carcinoma, epithelioid | Human | 3 |
| HEK293T | Kidney | Tumor | Human | 5 |
| Hepa1-6 | Liver | Carcinoma | Mouse | 3 |
| HMVEC | Endothelial | Endothelial, microvascular | Human | 100 |
| HT-29 | Colon | Adenocarcinoma | Human | 3 |
| HUVEC | Umbilicus | Endothelial cells | Human | 100 |
| Jurkat | Blood | Leukemia, Acute T cell | Human | 10 |
| LLC-1 | Lung | Carcinoma | Mouse | 6 |
| LNCaP | Prostate | Carcinoma | Human | 5 |
| MM200 | Skin | Melanoma | Human | 5 |
| MCF-7 | Breast | Adenocarcinoma | Human | 2 |
| MDA-MB-231 | Breast | Adenocarcinoma | Human | 1 |
| MM-AN | Skin | Melanoma, metastatic | Human | 16 |
| MMC | Breast | Carcinoma | Mouse | 4 |
| MRC-5 | Lung, embryonic | Fibroblasts | Human | 1 |
| NB4 | Blood | Leukemia, acute promyelocytic | Human | 10 |
| PC12 | Adrenal gland | Pheochromocytoma | Rat | 20 |
| SKOV-3 | Ovary | Adenocarcinoma | Human | 15 |
| U-2 OS | Bone | Osteosarcoma | Human | 5 |
实验室常用的逆转录病毒(Retrovirus)、慢病毒(Lentivirus)、腺相关病毒(AAV)和腺病毒(Adenovirus)的主要特征之间的比较和差别参见下表。具体的特定病毒的一些特征和下表相比可能会有一定差异。
| Retrovirus | Lentivirus | AAV | Adenovirus | |
| Genome | ssRNA(+) | ssRNA(+) | ssDNA | dsDNA |
| Coat | Enveloped | Enveloped | Naked | Naked |
| Particle size | 90-100nm | 90-100nm | 20-30nm | 60-90nm |
| Genome size | 7-10kb | 9kb | 5kb | 38-39kb |
| Genome integration | Yes | Yes | No | No |
| Packaging capacity | 2.5-5kb | 2.5-6kb | 2.5-4.5kb | 3-8kb |
| Infection tropism | Dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells |
| Relative Transduction Efficiency | ND | 70% | 70% | 100% |
| Expression started | 48-72h | 48-72h | 72-96h | 24-48h |
| Expression duration | > 2 months | > 2 months | > 6 months | 3-4 weeks |
| Expression level | Medium | Medium | Medium | High |
| Immune response | Low | Low | Very low | High |
| In vivo safety | Medium | Medium | High | Low |
| Titer before concentration (IFU/ml) | 106 | 107 | 1011 | 107 |
| Titer after concentration (IFU/ml) | ND | 108 | 0.5-1×1013 | 1010 |
| Able to obtain high MOI | No (≤ 10 copies integrated) | No (≤ 10 copies integrated) | Yes | Yes |
| Biosafety level | BSL-2 | BSL-2 | BSL-1 | BSL-2 |
本产品的滴度不低于10^9TU/ml,适合细胞实验或活体动物实验。TU, transduction unit,即转导单位。TU通过本产品梯度稀释后感染HEK293T细胞后测定获得,能让一个HEK293T细胞呈现绿色荧光的活力单位定义为1TU。MOI (Multiplicity of Infection)是病毒感染细胞时,病毒数量与细胞数量的比值。使用10^9TU/ml的本产品,如果按照5 MOI感染6孔板的细胞,每孔50万细胞计算,100μl共可以感染40个孔;如果按照5 MOI感染24孔板的细胞,每孔10万细胞计算,100μl共可以感染200个孔。如果MOI值提高,相应可以感染的孔数会减少;如果MOI值下调,相应可以感染的孔数会增加。
包装清单:| 产品编号 | 产品名称 | 包装 |
| C4007-100μl | Lenti-CMV-CD9-EGFP (10^9TU/ml,外泌体示踪用) | 100μl |
| C4007-500μl | Lenti-CMV-CD9-EGFP (10^9TU/ml,外泌体示踪用) | 100μl×5 |
| - | 说明书 | 1份 |
-80℃保存,一年有效。-20℃保存,1-2个月内有效。4℃保存,一周内有效。
注意事项:反复冻融会降低病毒滴度,如有必要请在收到本产品后分装保存。分装时必须在冰浴上进行。病毒融解后,如果在一周内使用,可以放于4℃,但须注意4℃存放时间越长,滴度下降越明显。如果-80℃保存时间超过一年,可能会导致滴度下降,此时建议重新测定病毒滴度。
本产品使用前请仔细阅读附录1《慢病毒使用安全规范》。本产品生物安全等级为Biosafety Level 2 (BSL-2),在按照常规的微生物实验操作要求进行操作(Standard microbiological practices)的基础上,还需要注意限制接触、生物危害提示、显著的警示标识、并制定相应的安全规范。
病毒操作中应注意有效防护,绝对禁止在生物安全柜内有任何皮肤直接暴露的情况。实验完成后,请及时清洗双手。严禁直接接触病毒,如意外接触,请及时用清水冲洗,并适当用70%乙醇对皮肤进行消毒。
任何接触过病毒的材料、试剂、样品,应经消毒处理,可以采用1%的SDS溶液、或84消毒液(1:20)浸泡30分钟以上,或121℃高压灭菌30分钟。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
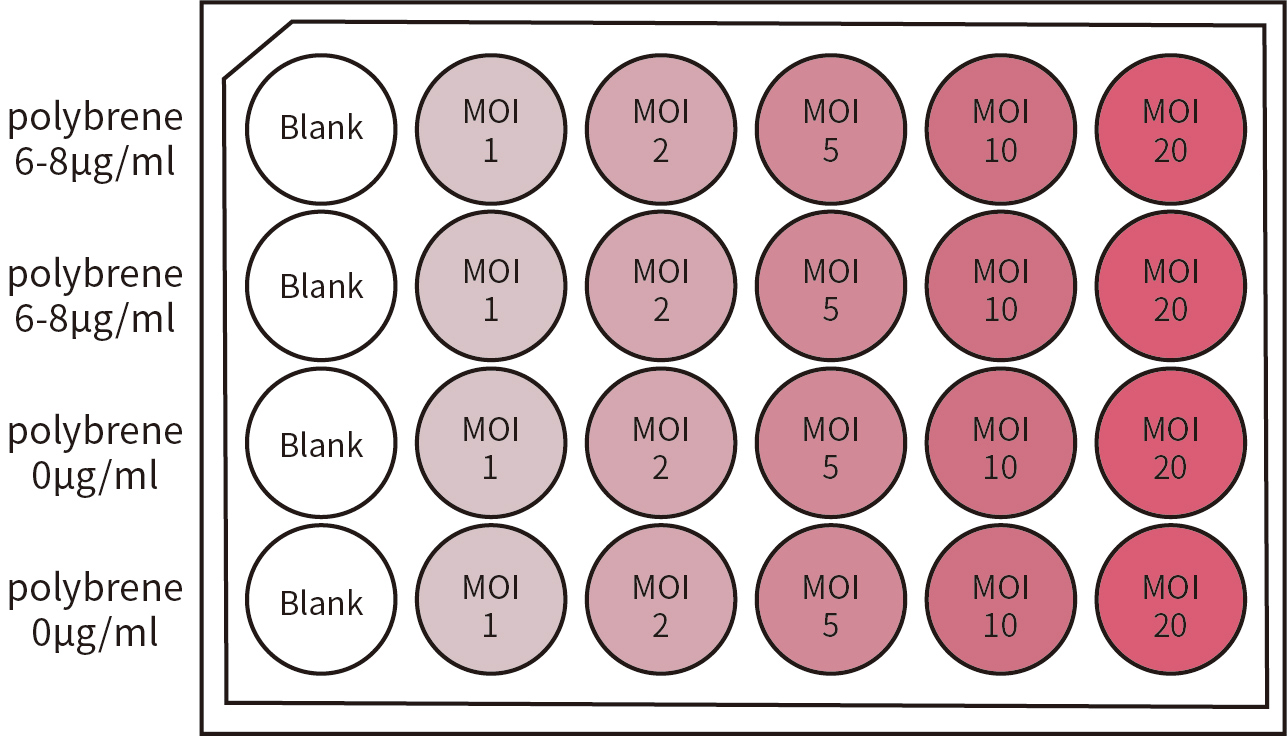 图4.细胞感染MOI值预实验设计分组。MOI依次设置为:1、2、5、10、20。并同时设置加含6-8μg/ml polybrene的培养液实验组,不含polybrene的培养液实验组及Blank组(空白对照组)。Blank组可作为参照,以检验细胞生长状态。本图中实验设置仅供参考,用户应根据实际情况自行适当调整。
图4.细胞感染MOI值预实验设计分组。MOI依次设置为:1、2、5、10、20。并同时设置加含6-8μg/ml polybrene的培养液实验组,不含polybrene的培养液实验组及Blank组(空白对照组)。Blank组可作为参照,以检验细胞生长状态。本图中实验设置仅供参考,用户应根据实际情况自行适当调整。| BSL | Agents | Practices | Primary Barriers andSafety Equipment | Facilities(Secondary Barriers) |
| 1 | Not known to consistently cause diseases in healthy adults | Standard microbiological practices | ■ No primary barriers required. ■ PPE: laboratory coats and gloves; eye, face protection, as needed |
Laboratory bench and sink required |
| 2 | ■ Agents associated with human disease ■ Routes of transmission include percutaneous injury, ingestion, mucous membrane exposure |
BSL-1 practice plus: ■ Limited access ■ Biohazard warning signs ■ “Sharps” precautions ■ Biosafety manual defining any needed waste decontamination or medical surveillance policies |
Primary barriers: ■ BSCs or other physical containment devices used for all manipulations of agents that cause splashes or aerosols of infectious materials ■ PPE: Laboratory coats, gloves, face and eye protection, as needed |
BSL-1 plus: ■ Autoclave available |
| 3 | Indigenous or exotic agents that may cause serious or potentially lethal disease through the inhalation route of exposure | BSL-2 practice plus: ■ Controlled access ■ Decontamination of all waste ■ Decontamination of laboratory clothing before laundering |
Primary barriers: ■ BSCs or other physical containment devices used for all open manipulations of agents ■ PPE: Protective laboratory clothing, gloves, face, eye and respiratory protection, as needed |
BSL-2 plus: ■ Physical separation from access corridors ■ Self-closing, double-door access ■ Exhausted air not recirculated ■ Negative airflow into laboratory ■ Entry through airlock or anteroom ■ Hand washing sink near laboratory exit |
| 4 | ■ Dangerous/exotic agents which post high individual risk of aerosol-transmitted laboratory infections that are frequently fatal, for which there are no vaccines or treatments ■ Agents with a close or identical antigenic relationship to an agent requiring BSL-4 until data are available to redesignate the level ■ Related agents with unknown risk of transmission |
BSL-3 practices plus: ■ Clothing change before entering ■ Shower on exit ■ All material decontaminated on exit from facility |
Primary barriers: ■ All procedures conducted in Class III BSCs or Class I or II BSCs in combination with full-body, air-supplied, positive pressure suit |
BSL-3 plus: ■ Separate building or isolated zone ■ Dedicated supply and exhaust, vacuum, and decontamination systems ■ Other requirements outlined in the text |









 微信在线咨询
微信在线咨询












